There are more than 300 approved cancer drugs, all which work – to some extent – for some cancer patients but not for all. This causes great difficulty for the physician to decide which medicine the patient should receive. If the patient is given the wrong medicine, the tumor continues to grow, time is lost and the chances of a cure risk decreasing. Cancer drugs are also often associated with serious side effects.
General statistics are the first step in determining which drug should be used, but the rate of ineffective treatments is still as high as 75 percent in difficult-to-treat cancers. For more patients to receive the right medicine right from the start, more effective predictive models are needed that captures the individual characteristics of patients and their tumors. That’s what CHOSA is all about.
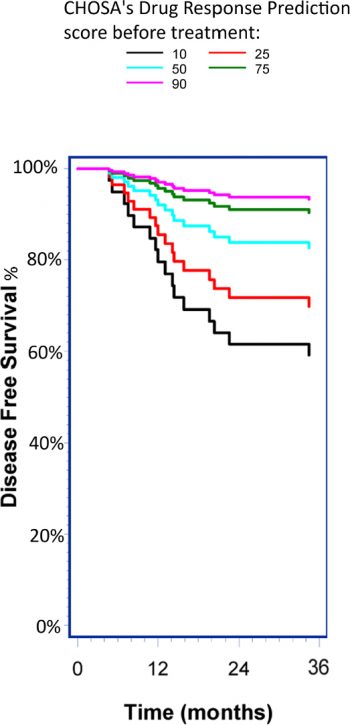
iCIP™ (LiPlaCis® and DRP® together) consists of two innovative technologies that can potentially contribute to more effective cancer care. Although the technologies can be used individually, the greatest benefit is found in using them together. The first technology is CHOSA’s drug candidate LiPlaCis®, which is an improved formulation of the already approved chemotherapy drug cisplatin. Cisplatin has been one of the most commonly used drugs in cancer treatment since it was first approved in 1978. It is estimated that 10-20 percent of all cancer patients receive some form of cisplatin at some point during their treatment and there is currently nothing to indicate that this will decrease, but rather that the usage appears to be increasing. Like the best drugs on the market, however, cisplatin only works in about 25 percent of cancer patients, and that’s not something that has changed over time. Like other cancer drugs, cisplatin also has several serious side effects. CHOSA’s candidate LiPlaCis® is a new formulation of cisplatin that, through a smart liposome carrier, targets sPLA2 – an enzyme found in tumors. Upon contact with the enzyme, the liposome bubble breaks down, and the encapsulated cisplatin is released directly into the tumor. The goal is that LiPlaCis® will be more local, effective and have fewer side effects compared to other formulations of cisplatin. The other technology is CHOSA’s drug response predictor – DRP®, a so-called “companion diagnostic” to predict whether a patient will benefit from the cisplatin or not. From the patient’s existing biopsy, the DRP® technology produces a rating of the patient’s suitability for cisplatin treatment, based on gene expression from the 205 genes known to most influence sensitivity and resistance to cisplatin. iCIP™ is thus the combination of being able to identify in advance whether a patient will benefit from the cisplatin treatment or not, and then treat the patient with higher efficiency and less toxicity than existing alternatives. CHOSA recently received positive Phase 2b clinical data showing that patients selected with DRP® respond better to treatment, have longer progression-free survival and possibly longer overall survival than patients who had a low DRP score.